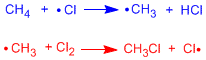
Propagation Stages
Let's represent in an energy vs reaction coordinate diagram the two propagation stages for the halogenation of methane.
Energy Diagram
The first propagation stage is the rate-limiting step of the process, having the highest activation energy. The diagram represents reactants, products, intermediates, and transition states for the radical halogenation of methane.
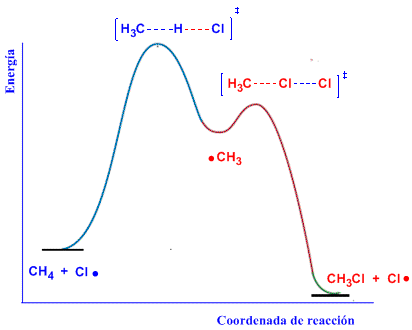
In the first transition state, the H-Cl bond is formed and the C-H bond is broken. In the second transition state, the C-Cl bond is formed and the Cl-Cl bond is broken.
