Estructura del ciclopropano
Estructura del ciclopropano
El ángulo internuclear C-C-C en el ciclopropano es de 60º, muy inferior al ángulo natural del enlace entre C(sp3) que se sitúa en los 109.5º. En la práctica los enlaces C-C en el ciclopropano se doblan hacia el exterior para permitir el solapamiento, dando lugar a unos enlaces más largos y débiles que los correspondientes a los alcanos normales. Otro factor que contribuye a la tensión en el ciclopropano es el eclipsamiento entre hidrógenos.
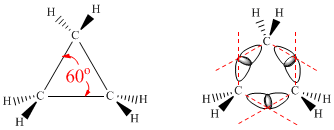
El ciclopropano se dispone plano, presenta 6 seis hidrógenos eclipsados y una importante tensión angular. Los enlaces sigma carbono-carbono están ligeramente doblados hacia el exterior, debido a la importante tensión. Estos enlaces curvos se denominan banana.
 ¿Te cuesta entender la Química Orgánica?
¿Te cuesta entender la Química Orgánica?