Cyclopentane Structure
The planar structure of cyclopentane has bond angles of about 108º, which are very close to the 109.5º of sp³ carbons, and should not present significant strain. However, in a planar structure, cyclopentane has ten hydrogen eclipses that would give it an approximate strain energy of 10 kcal/mol.
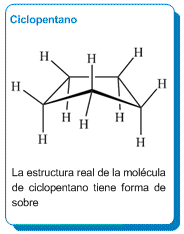
Cyclopentane Spatial Arrangement
The actual structure of the cyclopentane molecule is envelope-shaped, which reduces hydrogen eclipses and lowers the ring strain of the molecule.
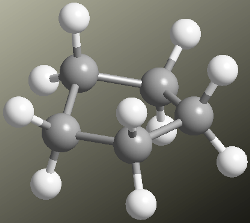
Cyclopentane Molecular Model
Cyclopentane is arranged in an envelope shape, taking one of its carbons out of the plane, which reduces the number of eclipses, making cyclopentane a practically strain-free molecule.
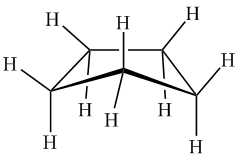
Video
Additional Notes
* Cyclopentane is a relatively stable molecule due to its conformation.
* Cyclopentane undergoes substitution reactions more readily than cyclopropane.
* Cyclopentane is found in small amounts in petroleum.