 ¿Te cuesta entender la Química Orgánica?
¿Te cuesta entender la Química Orgánica?
Cursos de Química Orgánica para los Grados en Química, Ingeniería Química, Biotecnología y Farmacia de las Universidades españolas.
Material específico para cada Universidad con teoría, ejercicios y exámenes resueltos en vídeo, creado por Germán Fernández. Soporte para dudas por WhatsApp.
Más información en www.foroquimico.com
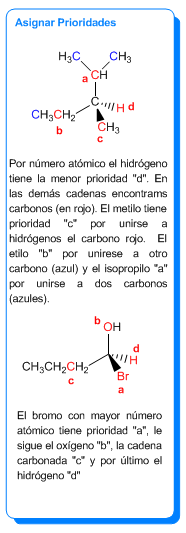 Reglas para nombrar enantiómeros
Reglas para nombrar enantiómeros
Para dar notación R/S a un centro quiral es necesario asignar prioridades a los sustituyentes mediante las siguientes reglas:
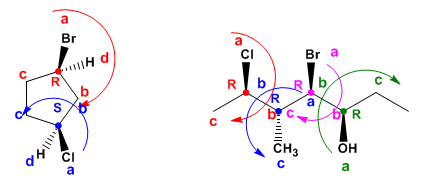
- Regla 1: Las prioridades de los átomos unidos al quiral se dan por números atómicos. En el caso de isótopos, tiene prioridad el de mayor masa atómica.
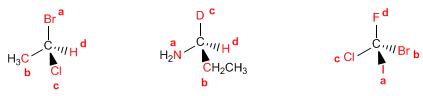
Las prioridades se dan por orden de número atómico de los átomos unidos directamente al carbono asimétrico (dibujados en rojo)
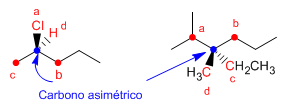
- Regla 2: Cuando dos o más sustituyentes unidos al centro quiral tengan la misma prioridad, se continua comparando las cadenas átomo a átomo hasta encontrar un punto de diferencia.
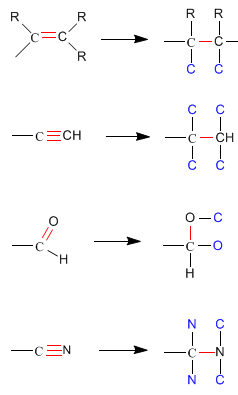
- Regla 3: Los enlaces dobles y triples se desdoblan considerándolos como si fueran enlaces sencillos.
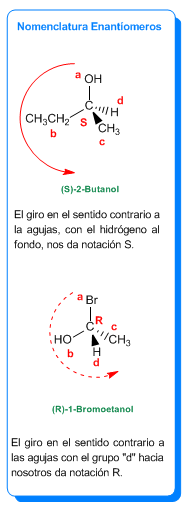
Para asignar notación R/S seguimos el orden de prioridades a, b, c de los sustituyentes. Si esta sucesión se realiza en el sentido de las agujas del reloj se dice que el centro es R (rectus, latín derecha). Si se sigue el sentido contrario a las agujas al recorrer las prioridades a, b, c se dice que es S
(sinester, latín izquierda). Esta regla sólo es válida cuando el grupo d está hacia el fondo del plano (enlace a trazos), si d sale hacia nosotros (cuña) la notación es la contraria (R giro a la izquierda, S giro a la derecha).
YouTube | Miembros del canal Hazte miembro del canal para acceder a todos los vídeos Vídeos para miembros