 ¿Te cuesta entender la Química Orgánica?
¿Te cuesta entender la Química Orgánica?
Cursos de Química Orgánica para los Grados en Química, Ingeniería Química, Biotecnología y Farmacia de las Universidades españolas.
Material específico para cada Universidad con teoría, ejercicios y exámenes resueltos en vídeo, creado por Germán Fernández. Soporte para dudas por WhatsApp.
Más información en www.foroquimico.com
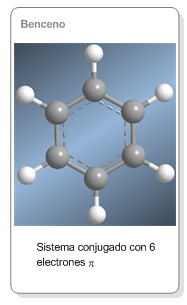 Aromaticidad del benceno
Aromaticidad del benceno
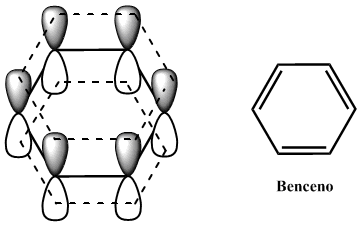
El benceno es un sistema cíclico de 6 electrones π deslocalizados. Está formado por 6 carbonos con hibridación sp2 cuyos orbitales p solapan formando una nube que permite la deslocalización de los electrónes p y confiere al benceno una gran estabilidad.
Reglas de Hückel
Esta energía de estabilización, llamada aromaticidad, está presente en otras moléculas que podemos identificar siguiendo las reglas de Hückel:
Regla 1.- Un sistema aromático debe ser cíclico y plano (sin carbonos sp3 en el ciclo)
Regla 2.- Existe conjugación cíclica de los dobles enlaces.
Regla 3.- El número de electrones p del sistema aromático cumple la fórmula 4n + 2, con n = 0, 1, 2, 3 ......
YouTube | Miembros del canal Hazte miembro del canal para acceder a todos los vídeos Vídeos para miembros