 ¿Te cuesta entender la Química Orgánica?
¿Te cuesta entender la Química Orgánica?
Cursos de Química Orgánica para los Grados en Química, Ingeniería Química, Biotecnología y Farmacia de las Universidades españolas.
Material específico para cada Universidad con teoría, ejercicios y exámenes resueltos en vídeo, creado por Germán Fernández. Soporte para dudas por WhatsApp.
Más información en www.foroquimico.com
Regla 1. Los alquenos son hidrocarburos que responden a la fórmula CnH2n. Se nombran utilizando el mismo prefijo que para los alcanos (met-, et-, prop-, but-....) pero cambiando el sufijo -ano por -eno.
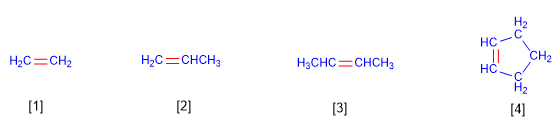
[1] Eteno
[2] Propeno
[3] 2-Buteno
[4] Ciclopenteno
Regla 2. Se toma como cadena principal la más larga que contenga el doble enlace. En caso de tener varios dobles enlaces se toma como cadena principal la que contiene el mayor número de dobles enlaces (aunque no sea la más larga)
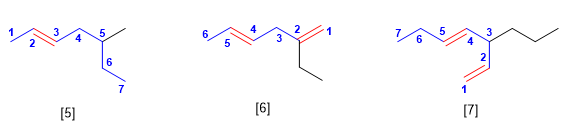
[5] 5-Metilhept-2-eno
[6] 2-Etilhexa-1,4-dieno
[7] 3-Propilhepta-1,4-dieno
Regla 3. La numeración comienza por el extremo de la cadena que otorga al doble enlace el localizador más bajo posible. Los dobles enlaces tienen preferencia sobre los sustituyentes
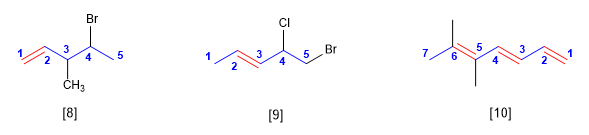
[8] 4-Bromo-3-metilpent-1-eno
[9] 5-Bromo-4-cloropent-2-eno
[10] 5,5-Dimetilhepta-1,3,5-trieno
Regla 4. Los alquenos pueden existir en forma de isómeros espaciales, que se distinguen con la notación cis/trans.
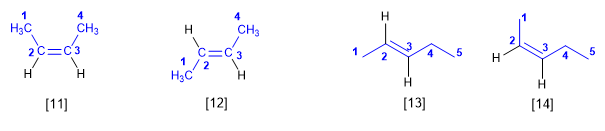
[11] cis-2-buteno
[12] trans-2-buteno
[13] trans-2-penteno
[14] cis-2-penteno
La notación cis- indica grupos iguales al mismo lado del doble enlace. La notación trans- se emplea cuando los grupos del mismo tipo quedan a lados opuestos del alqueno.
Se escribe en cursiva, siempre con minúscula y separado del nombre por un guión.
YouTube | Miembros del canal Hazte miembro del canal para acceder a todos los vídeos Vídeos para miembros