Rule 1. The main chain chosen should be the longest one containing the -OH group.
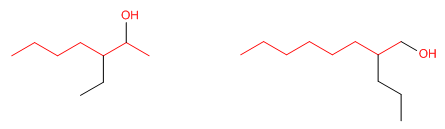
Rule 2. Number the main chain so that the -OH group receives the lowest possible locator. The hydroxyl group takes precedence over carbon chains, halogens, double bonds, and triple bonds.
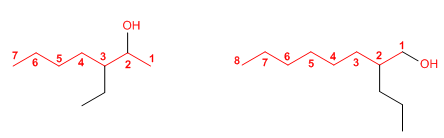
Rule 3. The name of the alcohol is formed by changing the ending -o of the alkane with the same number of carbons to -ol.
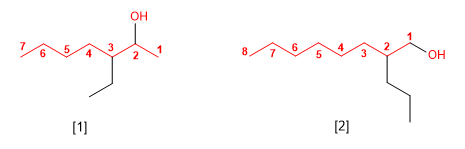
[1] 3-Ethylheptanol
[2] 2-Propyloctanol
Rule 4. When the molecule contains functional groups of higher priority, the alcohol becomes merely a substituent and is named hydroxy-. Higher priority groups include carboxylic acids, anhydrides, esters, acyl halides, amides, nitriles, aldehydes, and ketones. (see Table 1)
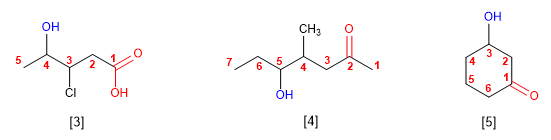
[3] 3-Chloro-4-hydroxypentanoic acid
[4] 5-Hydroxy-4-methylheptanone
[5] 3-Hydroxycyclohexanone
Rule 5. The -OH group takes precedence over alkenes and alkynes. The numbering assigns the lowest locator to the -OH, and the molecule's name ends in -ol.
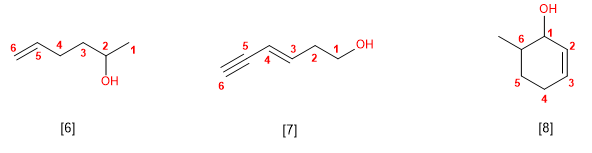
[6] Hex-5-en-2-ol
[7] Hex-3-en-5-in-1-ol
[8] 6-Methylcyclohex-2-en-1-ol